Tokyo Tech News
Tokyo Tech News
Published: May 17, 2023
The biofilm-forming bacteria E. coli adheres firmly to hydrophobic and hydrophilic protein-adsorbing self-assembling monolayers (SAMs) and weakly to hydrophilic protein-resisting SAMs, uncovered a recent study by Tokyo Tech researchers. These findings on how surface chemistry can influence the adhesion of bacterial cells and, in turn, biofilm formation could open doors to bacteria-resistant surfaces and antibiofouling coatings for biomedical and industrial devices.
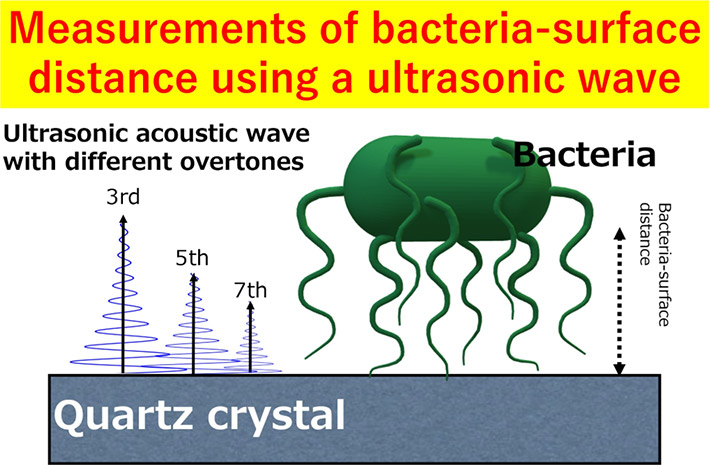
Bacterial biofilms are like a double-edged sword. On one hand, they have proven valuable for electricity generation and degradation of toxic materials in water and soil but on the other hand, they are a nuisance when formed inside drinking water pipes, on contact lenses and on biomedical devices. To form biofilms, the bacterial cells first attach to the surface of materials via weak and reversible bonds. Once these bonds mature, the cells release polymeric substances that result in stable and irreversible biofilms.
Decoding the mechanism underlying the irreversible biofilm formation and its relation to the surface chemistry is key to unlocking biofilm prevention technologies. Over the years, scientists have proposed various techniques to narrow down the types of surfaces bacterial cells tend to adhere to. Unfortunately, the influence of surface chemistry and surface topography on biofilm formation have remained ambiguous.
Against this backdrop, a team of researchers led by Associate Professor Tomohiro Hayashi from Tokyo Institute of Technology (Tokyo Tech), Japan, set out to address this knowledge gap. In a recent breakthrough published in ACS Applied Bio Materials, the team investigated the adhesion mechanism of the E. coli bacteria on self-assembling monolayers (SAM) comprising different terminal groups, i.e., different surface chemistries, using optical microscopy and a technique called "quartz crystal microbalance with energy dissipation (QCM-D) monitoring."
Dr. Hayashi explains, "We chose QCM-D because it is an ultrasensitive device that can monitor adsorbed masses as small as nanograms using acoustic waves. Similarly, SAMs with tunable terminal groups functionalized on atomically flat QCM sensors were chosen since they can help untangle the influence of surface chemistry from that of substrate topography on bacterial attachment."
Studies have shown that bacterial attachment to a surface cannot be fully explained by the conventional mass loading theory. This is because the theory does not explain the positive and negative shifts in the resonant frequency (f ) caused by adsorption of additional mass onto the surface, which significantly impacts biofilm formation. Taking this complex behavior into account through a coupled-resonator model, the team examined the changes in f and the energy dissipation D of the QCM sensor oscillation and assessed their impact on bacterial attachment and viscoelasticity of the SAM surfaces with different functionalities.
The microscopy images along with QCM-D data revealed that E. coli adhered firmly to both hydrophobic (methyl-terminated) and hydrophilic protein-adsorbing (amine- and carboxy-terminated) SAMs (Figure 1a and b), forming dense bacterial films. In contrast, it attached weakly to hydrophilic protein-resisting SAMs (Figure 1c), such as oligo (ethylene glycol) and sulfobetaine, and formed sparse and dissipative biofilms.
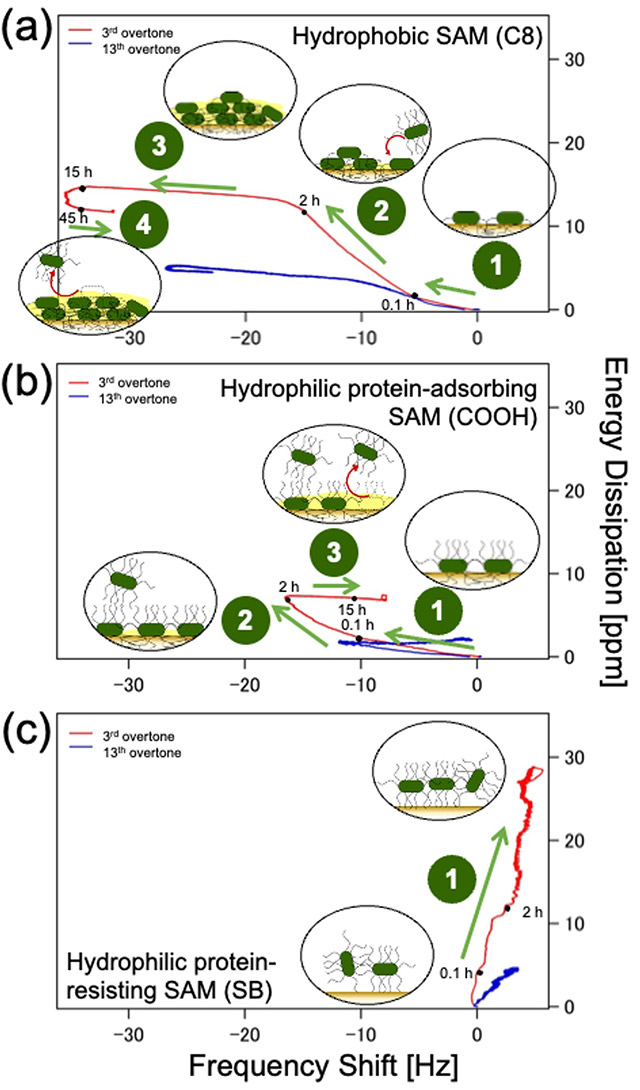
Figure 1. Bacterial adhesion on self-assembling monolayers (SAMs)
Plot of energy dissipation(D) vs shift in resonant frequency (f ) for hydrophobic and hydrophilic protein-adsorbing SAMs and hydrophilic protein-resisting SAM at n =3 (3rd overtone, red) and n = 13 (13th overtone, blue) to understand time progression of bacterial adhesion.
The team also observed a positive f shift for hydrophilic protein-resisting SAMs, due possibly to the fact that the bacterial appendages of E. coli played role in not only initiating contact with the surface and bonding but also in creating elastic spring-like links with the surface. Further, by estimating the distances of the bacterial cell bodies from different SAM surfaces based on the different acoustic wave penetration depths at each overtone (Figure 2), they provided a possible explanation of why bacterial cells adhere strongly to some surfaces and weakly to others.
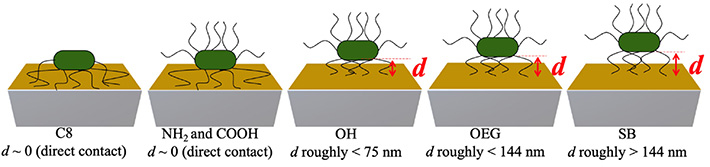
Figure 2. Illustration showing the estimated distances d of the bacterial cells for each SAM surface.
By estimating the acoustic wave penetration depths at each overtone, the distances of E. coli cells from each SAM surface could be determined, providing a clue on what makes bacterial cells adhere strongly to some surfaces and only weakly to others.
These valuable insights could help identify surfaces with higher probability of biofilm contamination as well as pave the way for contamination-resistant surfaces. "Our study can act as practical guide for determining which surfaces are prone to bacterial fouling as well as for designing prevention strategies such as bacteria-resistant surfaces and antibiofouling coatings," concludes Dr. Hayashi.
Reference
Authors : |
Glenn Villena Latag, Taichi Nakamura, Debabrata Palai, Evan Angelo Quimada Mondarte, and Tomohiro Hayashi |
Title : |
Investigation of Three-Dimensional Bacterial Adhesion Manner on Model Organic Surfaces Using Quartz Crystal Microbalance with Energy Dissipation Monitoring |
Journal : |
ACS Applied Bio Materials |
DOI : |
|
Affiliations : |
1 Department of Materials Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, Japan 2 School of Materials Science and Engineering, Nanyang Technological University, Singapore |
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
Further Information
Associate Professor Tomohiro Hayashi
School of Materials and Chemical Technology, Tokyo Institute of Technology
Email tomo@mac.titech.ac.jp
Tel +81-45-924-5400
Contact
Public Relations Division, Tokyo Institute of Technology
Email media@jim.titech.ac.jp
Tel +81-3-5734-2975